Delve into the realm of chemistry with our comprehensive Percent Composition by Mass Worksheet with Answers. This invaluable resource empowers you to unravel the secrets of chemical compositions, providing a solid foundation for understanding the intricate world of matter.
Embark on a journey of discovery as we delve into the intricacies of percent composition by mass, unraveling its significance in determining empirical and molecular formulas, and exploring its multifaceted applications in various scientific disciplines.
Percent Composition by Mass
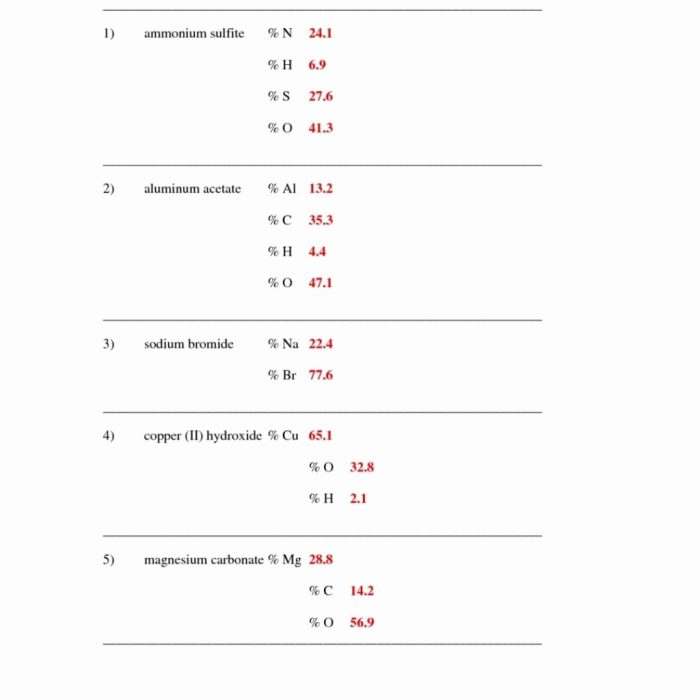
A percent composition by mass worksheet is a valuable tool for students to practice calculating the percentage of each element in a compound based on its mass.
An example of a percent composition by mass worksheet might include the following:
- A table with columns for the element symbol, mass, and percent composition
- A set of instructions for calculating the percent composition of each element
- Practice problems with answer keys
Calculating Percent Composition by Mass, Percent composition by mass worksheet with answers
To calculate the percent composition by mass of an element in a compound, the following steps are involved:
- Determine the mass of the element in the compound.
- Determine the total mass of the compound.
- Divide the mass of the element by the total mass of the compound and multiply by 100%.
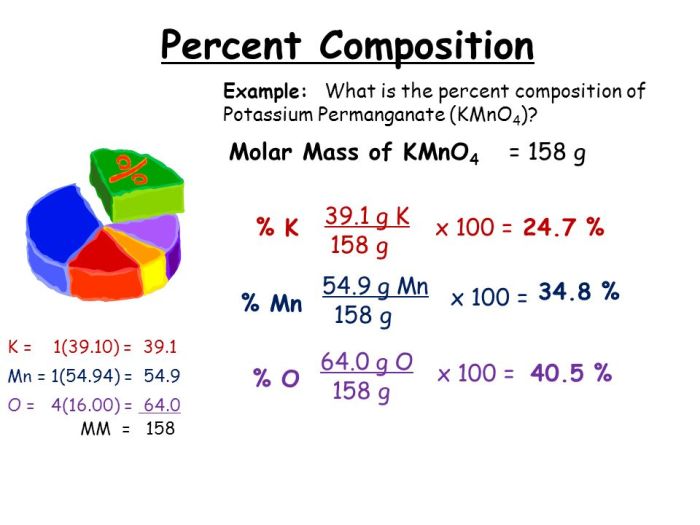
For example, to calculate the percent composition by mass of oxygen in water (H 2O), the following steps would be taken:
- The mass of oxygen in water is 16 amu.
- The total mass of water is 18 amu.
- The percent composition by mass of oxygen in water is (16 amu / 18 amu) x 100% = 88.89%.
Determining Empirical and Molecular Formulas
Percent composition by mass can be used to determine both empirical formulas and molecular formulas of compounds.
An empirical formula gives the simplest whole-number ratio of elements in a compound, while a molecular formula gives the actual number of atoms of each element in a molecule of the compound.
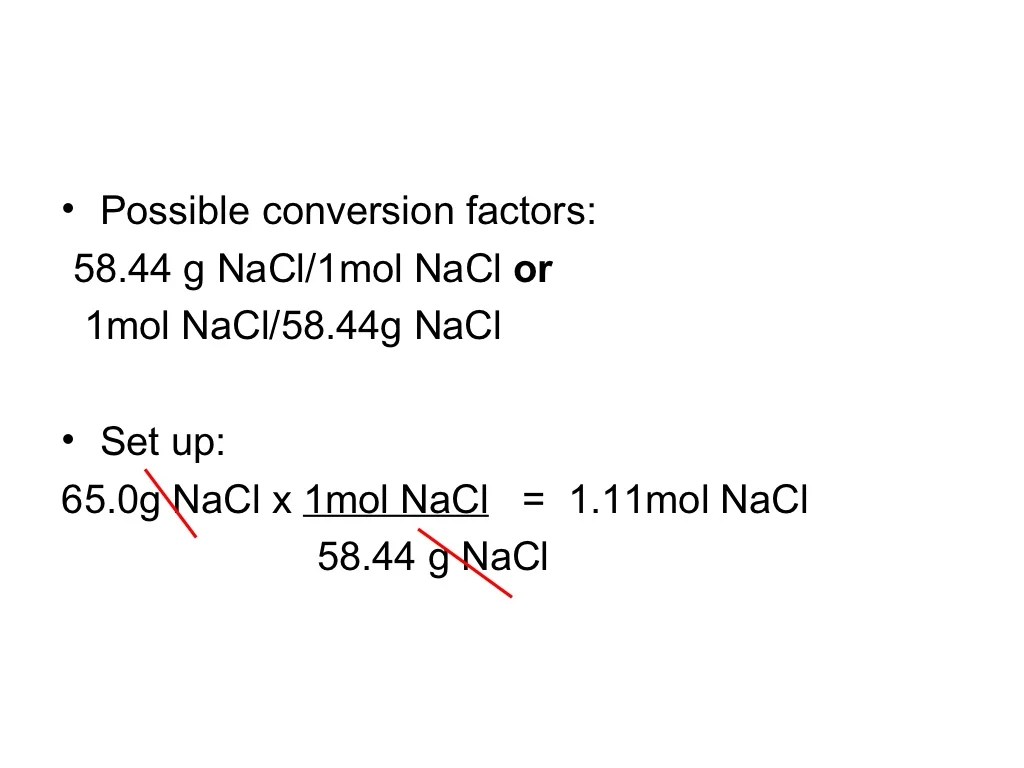
To determine the empirical formula of a compound, the following steps are involved:
- Convert the percent composition by mass of each element to grams.
- Convert the grams of each element to moles.
- Divide the number of moles of each element by the smallest number of moles.
- Multiply the result by the smallest whole number to obtain the simplest whole-number ratio.
To determine the molecular formula of a compound, the following steps are involved:
- Determine the empirical formula of the compound.
- Determine the molar mass of the empirical formula.
- Divide the molar mass of the compound by the molar mass of the empirical formula.
- Multiply the subscripts in the empirical formula by the result to obtain the molecular formula.
Stoichiometry and Percent Composition by Mass
Stoichiometry is the study of the quantitative relationships between reactants and products in chemical reactions.
Percent composition by mass can be used to calculate the stoichiometric ratio of reactants in a chemical reaction.
For example, if the percent composition by mass of oxygen in water is 88.89%, then the stoichiometric ratio of oxygen to hydrogen in water is 1:2.
This information can be used to calculate the mass of oxygen required to react with a given mass of hydrogen.
Applications of Percent Composition by Mass
Percent composition by mass has a wide range of applications in various fields, including:
- Chemistry: Determining the composition of compounds, calculating stoichiometric ratios, and understanding chemical reactions
- Materials science: Analyzing the composition of materials, determining their properties, and developing new materials
- Environmental science: Monitoring pollution levels, assessing the impact of human activities on the environment, and developing remediation strategies
- Medicine: Analyzing the composition of drugs, determining their dosage, and understanding their interactions with the body
Percent composition by mass is a fundamental tool for understanding the composition of matter and its applications in various fields.
FAQs: Percent Composition By Mass Worksheet With Answers
What is the purpose of a percent composition by mass worksheet?
A percent composition by mass worksheet provides a structured framework for calculating the percentage by mass of each element present in a compound.
How can percent composition by mass be used to determine empirical formulas?
By assuming a 100-gram sample of the compound, the percent composition by mass can be converted to grams of each element, which can then be used to determine the simplest whole-number ratio of elements in the empirical formula.
What is the relationship between stoichiometry and percent composition by mass?
Stoichiometry provides the mole ratios of reactants and products in a chemical reaction, which can be used to calculate the percent composition by mass of the products.