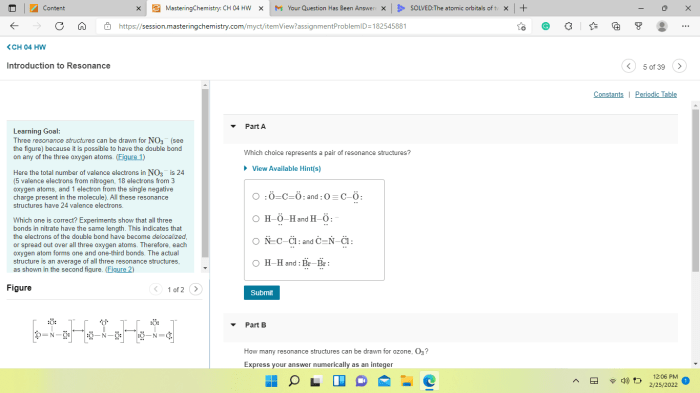
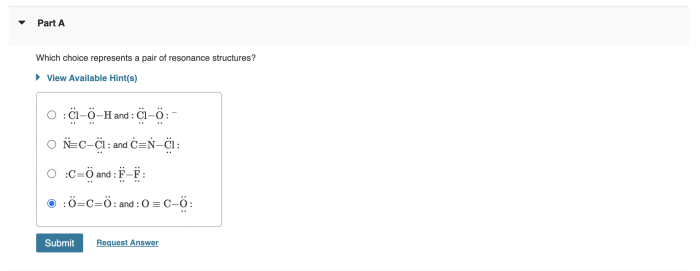
Which choice represents a pair of resonance structures – Delving into the realm of resonance structures, this exploration unveils the intricacies of these molecular representations, their significance in chemistry, and their profound impact on our understanding of molecular properties. Resonance structures, with their unique ability to depict the delocalization of electrons, provide valuable insights into the behavior and reactivity of chemical species.
As we embark on this journey, we will delve into the characteristics and guidelines for identifying resonance structures, examining pairs of these structures in both simple and complex molecules. Through illustrative examples and clear explanations, we will unravel the differences and similarities between resonance structures, gaining a deeper appreciation for their importance in predicting molecular properties and guiding chemical research.
1. Definition of Resonance Structures
Resonance structures are a representation of the delocalization of electrons within a molecule or polyatomic ion. They depict the distribution of electrons over several atoms or bonds, rather than being localized on a single atom or bond. Resonance structures are crucial in understanding the electronic structure and properties of molecules.
Resonance structures are not distinct chemical species but rather a way of representing the resonance hybrid, which is the actual state of the molecule. The resonance hybrid is a weighted average of the resonance structures, with the most stable resonance structure contributing the most to the hybrid.
2. Identifying Pairs of Resonance Structures
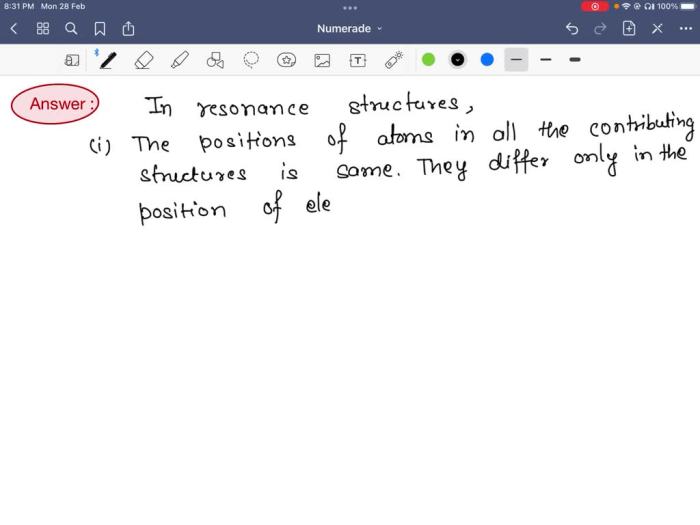
Pairs of resonance structures are two or more resonance structures that can be drawn for the same molecule or ion. They have the following characteristics:
- The same number of valence electrons in each structure
- The same connectivity of atoms
- Different placement of double bonds and lone pairs
To identify pairs of resonance structures, follow these steps:
- Draw the Lewis structure of the molecule or ion.
- Identify atoms or groups of atoms that can have multiple bonds or lone pairs.
- Move electrons to create alternative resonance structures while maintaining the same number of valence electrons and atomic connectivity.
- Evaluate the stability of each resonance structure and determine the major contributor to the resonance hybrid.
3. Examples of Pairs of Resonance Structures, Which choice represents a pair of resonance structures
Examples of pairs of resonance structures include:
- Benzene: The Kekule structures represent two resonance structures of benzene, with alternating single and double bonds.
- Carbon dioxide: The Lewis structure of CO 2can be represented by two resonance structures, with the double bond between carbon and oxygen in different orientations.
- Ozone: The Lewis structure of O 3can be represented by three resonance structures, each with a different distribution of double bonds and lone pairs.
4. Importance of Resonance Structures
Resonance structures play a significant role in understanding molecular properties:
- Stability:Resonance structures contribute to the stability of molecules by delocalizing electrons and lowering the overall energy of the system.
- Reactivity:Resonance structures can influence the reactivity of molecules by altering the distribution of electrons and making certain atoms or bonds more or less reactive.
- Molecular Geometry:Resonance structures can affect the molecular geometry by influencing the bond lengths and angles between atoms.
- Electronic Properties:Resonance structures can provide insights into the electronic properties of molecules, such as their ionization energy, electron affinity, and magnetic susceptibility.
Commonly Asked Questions: Which Choice Represents A Pair Of Resonance Structures
What are resonance structures?
Resonance structures are alternative representations of a molecule that depict the delocalization of electrons, providing a more accurate description of the molecule’s electronic structure.
How do you identify resonance structures?
Resonance structures must have the same number of electrons, atoms, and overall charge, and they must be connected by a single or double bond that can be broken and reformed.
What is the significance of resonance structures?
Resonance structures provide insights into the electronic distribution, reactivity, and stability of molecules, helping chemists predict their behavior and guide chemical research.