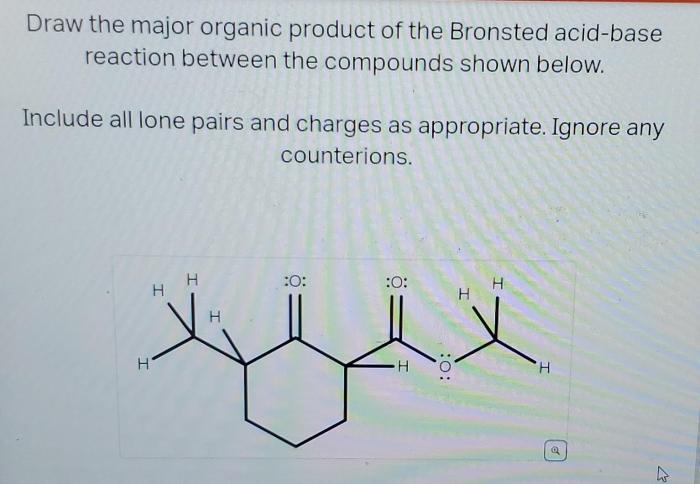
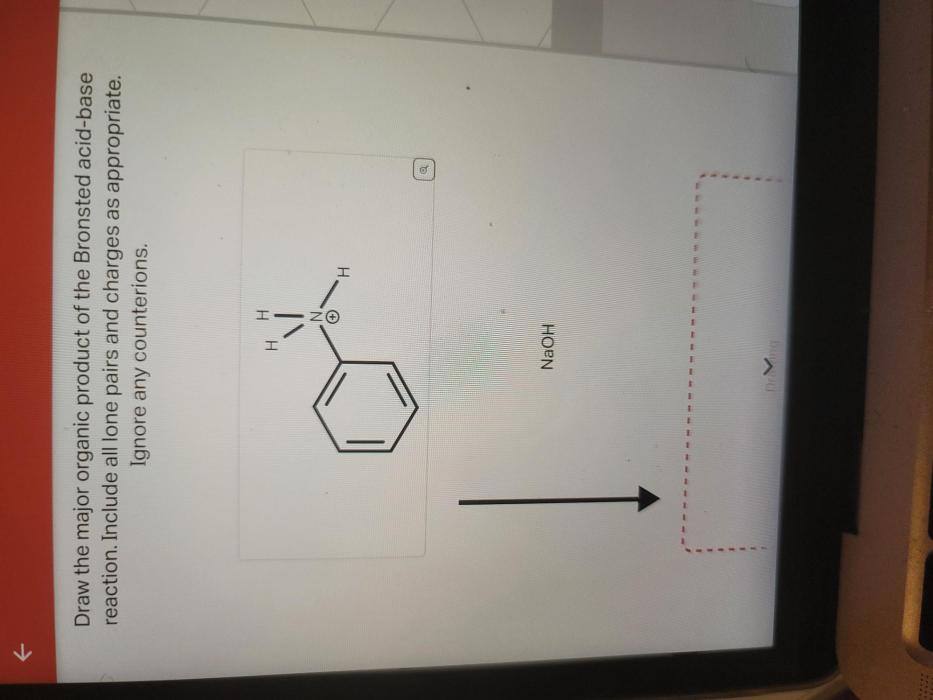
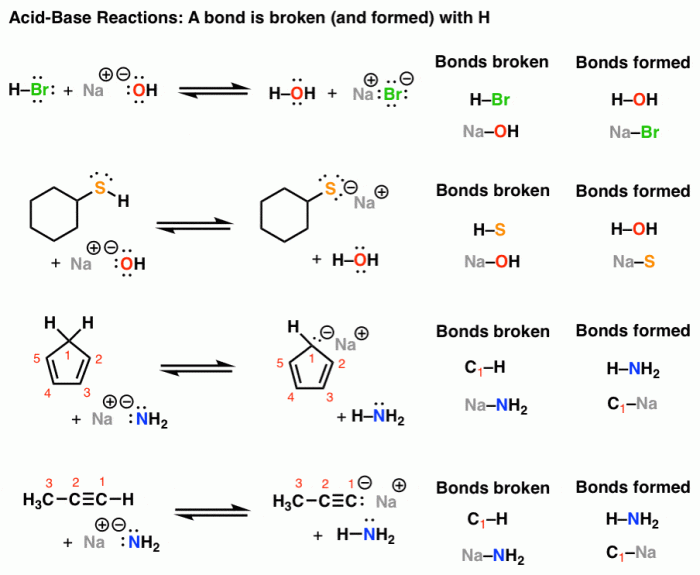
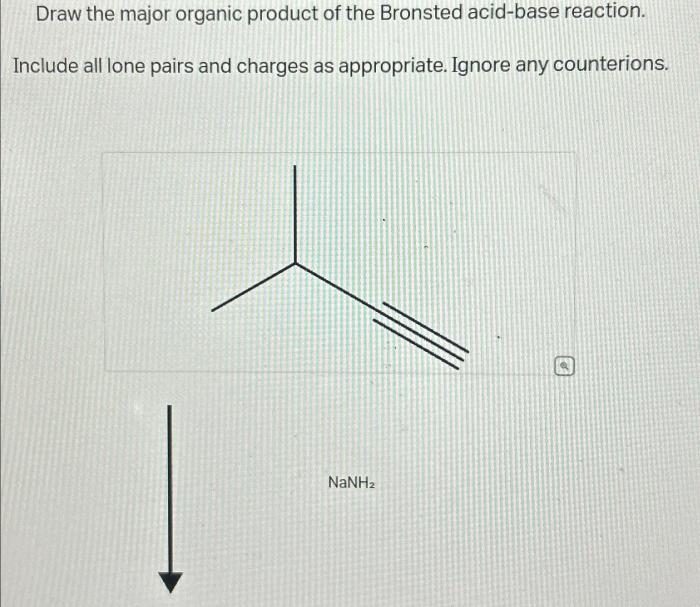
Draw the major organic product of the Bronsted acid-base reaction, a topic of paramount importance in organic chemistry, invites us to explore the fascinating world of acid-base interactions and their profound implications. This engaging discourse will delve into the intricacies of these reactions, empowering us to unravel their complexities and harness their potential.
As we embark on this intellectual journey, we will uncover the factors that govern the outcome of Bronsted acid-base reactions, enabling us to predict the major organic product with remarkable accuracy. Through a comprehensive analysis of the strength of acids and bases, we will gain insights into the mechanisms that drive these reactions, shedding light on the role of proton transfer in shaping their outcomes.
1. Introduction to Bronsted Acid-Base Reactions
Bronsted acid-base reactions are a fundamental type of chemical reaction involving the transfer of protons (H+ ions) between an acid and a base. An acid is a substance that donates protons, while a base is a substance that accepts protons.
The general form of a Bronsted acid-base reaction is:
Acid + Base → Conjugate Base + Conjugate Acid
For example, the reaction of hydrochloric acid (HCl) with sodium hydroxide (NaOH) is a Bronsted acid-base reaction:
HCl + NaOH → NaCl + H2O
In this reaction, HCl is the acid, NaOH is the base, NaCl is the conjugate base, and H2O is the conjugate acid.
2. Predicting the Major Organic Product
The outcome of a Bronsted acid-base reaction can be predicted based on the strength of the acid and base involved. The stronger the acid, the more readily it will donate protons, and the stronger the base, the more readily it will accept protons.
In general, the major organic product of a Bronsted acid-base reaction will be the conjugate acid of the weaker base and the conjugate base of the stronger acid.
For example, in the reaction of acetic acid (CH3COOH) with sodium hydroxide (NaOH), the major organic product will be sodium acetate (CH3COONa) and water (H2O).
This is because acetic acid is a weaker acid than HCl, and NaOH is a stronger base than H2O.
3. Mechanisms of Bronsted Acid-Base Reactions
Bronsted acid-base reactions occur through a mechanism involving the transfer of protons. The proton transfer can occur directly or through a solvent molecule.
In the direct mechanism, the acid and base come into direct contact, and the proton is transferred directly from the acid to the base.
In the solvent-mediated mechanism, the solvent molecule acts as an intermediary, accepting a proton from the acid and then transferring it to the base.
The solvent-mediated mechanism is more common in reactions involving weak acids and bases.
4. Applications of Bronsted Acid-Base Reactions, Draw the major organic product of the bronsted acid-base reaction
Bronsted acid-base reactions are used in a wide variety of applications, including:
- Organic synthesis
- Biological systems
- Acid-base titrations
- Buffer solutions
5. Advanced Concepts in Bronsted Acid-Base Reactions
In addition to the basic concepts discussed above, there are a number of more advanced concepts that can be applied to Bronsted acid-base reactions.
These concepts include:
- Conjugate acid-base pairs
- Equilibrium constants
- Acid-base catalysis
Popular Questions: Draw The Major Organic Product Of The Bronsted Acid-base Reaction
What are the key factors that influence the outcome of Bronsted acid-base reactions?
The strength of the acid and base involved play a crucial role in determining the outcome of these reactions.
How can we predict the major organic product based on the strength of the acid and base?
By considering the relative strengths of the acid and base, we can infer the direction of the reaction and predict the major organic product.
What is the role of proton transfer in Bronsted acid-base reactions?
Proton transfer is the central mechanism that drives these reactions, involving the transfer of a proton from the acid to the base.