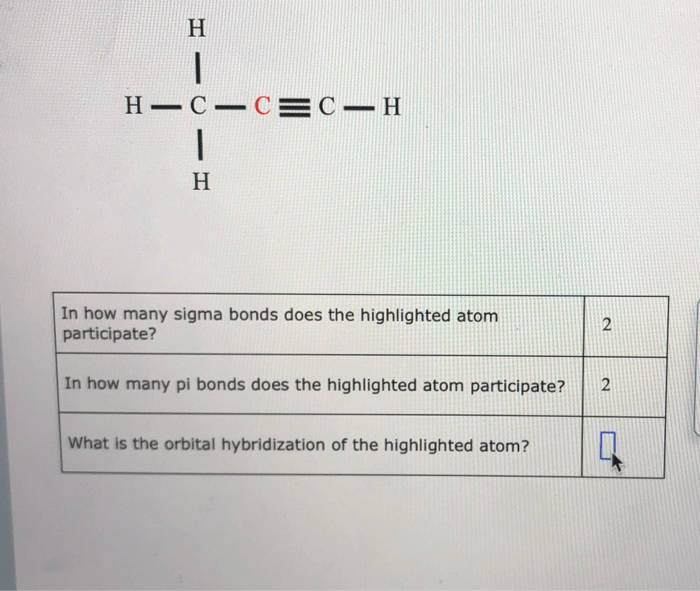
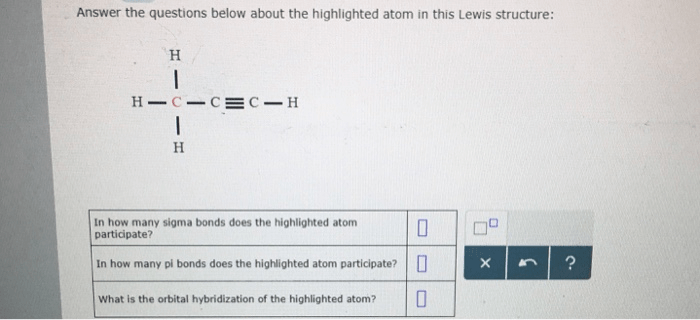
In how many sigma bonds does the highlighted atom participate? This question delves into the intricate world of molecular bonding, where sigma bonds form the backbone of stable and diverse chemical structures. Understanding sigma bond participation is crucial for unraveling the properties and behavior of countless compounds.
Delving deeper, we explore the methods used to determine the number of sigma bonds an atom participates in, including molecular orbital theory and hybridization. These tools provide valuable insights into the electronic structure and bonding patterns of molecules.
1. Atom’s Bonding Participation: In How Many Sigma Bonds Does The Highlighted Atom Participate
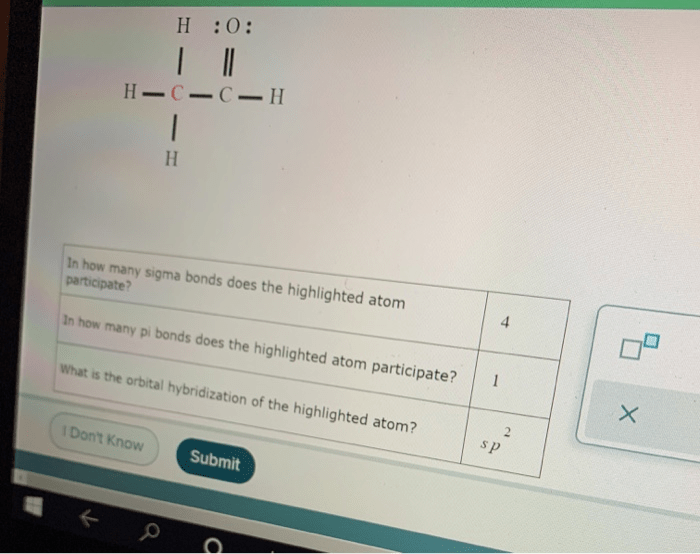
In chemistry, understanding the participation of atoms in sigma bonds is crucial for comprehending molecular structures and properties.
A sigma bond is a covalent bond formed by the head-to-head overlap of atomic orbitals. It represents the strongest type of covalent bond and is essential for the stability of molecules.
Identifying Sigma Bond Participation
Determining the number of sigma bonds an atom participates in involves examining its molecular orbital structure and hybridization.
- Molecular Orbital Theory:Sigma bonds are formed by the overlap of atomic orbitals that are symmetrical around the internuclear axis.
- Hybridization:Hybridization refers to the mixing of atomic orbitals to form new hybrid orbitals with specific geometries. The type of hybridization an atom undergoes determines the number and orientation of sigma bonds it can participate in.
Factors Influencing Sigma Bond Participation
Several factors influence the number of sigma bonds an atom can participate in:
- Electronegativity:Atoms with higher electronegativity tend to form fewer sigma bonds.
- Atomic Radius:Larger atoms can participate in more sigma bonds due to their increased orbital size.
- Hybridization:Different types of hybridization result in different numbers of sigma bonds. For example, sp3 hybridization allows an atom to participate in four sigma bonds, while sp2 hybridization allows three, and sp hybridization allows two.
Consequences of Sigma Bond Participation
The participation of atoms in sigma bonds has significant consequences for molecular geometry and stability:
- Molecular Geometry:The number of sigma bonds an atom participates in determines the shape of the molecule.
- Stability:Sigma bonds contribute to the overall stability of molecules by providing strong covalent bonds between atoms.
Applications of Sigma Bond Participation Analysis, In how many sigma bonds does the highlighted atom participate
Analyzing sigma bond participation finds practical applications in various fields of chemistry:
- Drug Design:Understanding sigma bond participation helps design drugs with specific molecular geometries and properties.
- Materials Science:Sigma bond analysis guides the development of new materials with tailored properties.
FAQs
What are sigma bonds?
Sigma bonds are covalent bonds formed by the head-to-head overlap of atomic orbitals, resulting in a cylindrical electron density distribution along the internuclear axis.
How can we determine the number of sigma bonds an atom participates in?
The number of sigma bonds an atom participates in can be determined using molecular orbital theory or hybridization. Molecular orbital theory involves analyzing the overlap of atomic orbitals to form molecular orbitals, while hybridization considers the mixing of atomic orbitals to form hybrid orbitals with specific geometries.